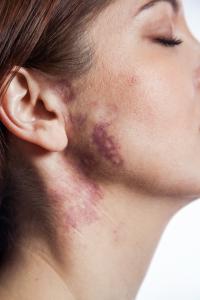
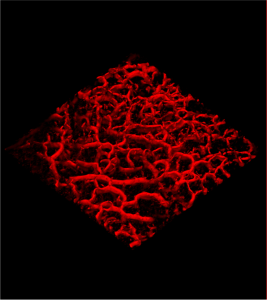
New Hope for Port Wine Stain Patients from a Novel Image-Guided Treatment
New 'OCT' scanning can image and measure the tiny blood vessels in the skin, to help guide laser treatment of disfiguring skin lesions.
The novel technique, known as OCT image-guided treatment, utilizes a high-resolution laser scanner to detect and image the tiny blood vessels in the skin, so that the depth, diameter and density of these vessels can be accurately characterized. This information is crucial for optimizing the settings for laser or Broadband Light (BBL) treatments, so that diseased and abnormal vessels are destroyed with maximum efficiency. This holds the potential for improved clearance of the condition and with fewer treatment sessions.
“We are enthusiastic about the results we are seeing with our VivoSight OCT-guided treatments of Port Wine Stain birthmarks”, said Dr Roy Geronemus of the Laser & Skin Surgery Center of New York, and who has been testing the VivoSight OCT system, “This new technology has great potential to change therapies for vascular conditions. The first results are very encouraging, and we are evaluating the technology to guide treatment of other conditions beyond Port Wine Stains.”
Optical Coherence Tomography, or OCT, is used by dermatologists for precision imaging of skin morphology and vascular patterns, using a hand-held probe. Michelson Diagnostics has now released a new version of the VivoSight OCT scanner which can automatically extract critical vessel parameters and display them in real time, to help clinicians choose the optimal treatment settings on their laser or BBL device.
“I use the VivoSight system in my practice all the time and have been working with the technology for over three years and we are finding VivoSight OCT to be useful for a variety of conditions.”, said Dr. Jill Waibel, of Miami Dermatology & Laser Institute. “Not only do we use OCT to guide the treatment of patients with vascular skin conditions, but also to guide treatment of vessels in scars, resulting in better and faster results. The new vessel software on the device will make this straightforward and intuitive.”
UK-based Michelson Diagnostics Ltd (Maidstone, Kent) is collaborating with Sciton Inc. (Palo Alto, CA), the leading US manufacturer of medical and aesthetic lasers and light source technologies, to bring to market a range of OCT-image-guided skin treatments.
Jon Holmes, CEO of Michelson Diagnostics, remarks: “We are working with Sciton and with Key-Opinion-Leader clinicians in multiple institutions in the USA and globally, to develop new OCT image-guided laser and light-treatment capabilities for many skin conditions, including vascular problems, scars, non-melanoma skin cancer and more. Our vision is a new paradigm in which skin specialists are no longer ‘flying blind’ when they treat, but instead they can apply all the power of imaging, just like MRI, CT and Ultrasound are used, to guide treatments for a variety of conditions”.
The VivoSight OCT Scanner is being demonstrated this week at the World Congress of Dermatology, Milan, booth no. B13 (Sciton).
References:
Waibel JS, Holmes J, Rudnick A, Woods D, Kelly KM. Angiographic optical coherence tomography imaging of hemangiomas and port wine birthmarks. Lasers in surgery and medicine. 2018 Mar 22.
Waibel A, Rudnick AC, Wulkan AJ, Holmes JD, The Diagnostic Role of OCT in Measuring the Depth of Burn and Traumatic Scars for More Accurate Laser Dosimetry: A pilot Study, J Drugs in Derm.2016. Nov;15(11)
About Michelson Diagnostics’ VivoSight OCT scanner:
Michelson Diagnostics’ innovative VivoSight Dx OCT scanner has FDA 510(k) clearance for use by trained clinicians for scanning skin lesions as an aid to assessing the patient’s condition. Using novel patented laser optics in an easy to use hand-held probe, it provides real-time, high-resolution subsurface images of skin tissue that reveal valuable information useful to the dermatologist.
About Michelson Diagnostics (www.vivosight.com)
Michelson Diagnostics is headquartered in Maidstone, UK, and has sales and support subsidiaries in Germany and in California, USA. Founded in 2006 by Jon Holmes and colleagues, Michelson Diagnostics designs, manufactures and supports highly innovative imaging instruments using multi-beam optical coherence tomography technology. Michelson Diagnostics is the world’s leading supplier of Optical Coherence Technology (OCT) devices for dermatology, and operates globally with agents in other countries including China, Japan, Australia and Poland.
About Sciton (www.sciton.com)
Sciton, Inc., located in Palo Alto, California, is committed to providing best-in-class laser and light solutions for medical professionals who want superior durability, performance and value. Sciton offers best-in-class medical devices for laser-assisted lipolysis, fractional and full-coverage skin resurfacing, hair removal, phototherapy, wrinkle reduction, treatment of vascular and pigmented lesions, scar reduction and acne. For more information, and a complete list of Sciton systems, visit www.Sciton.com.
For clinical use in the US FDA 510(k) K093520 applies:
VivoSight is a Multi-Beam Optical Coherence Tomography (OCT) system indicated for use in the two-dimensional, cross-sectional, real-time imaging of external tissues of the human body. This indicated use allows imaging of tissue microstructure, including skin, to aid trained and competent clinicians in their assessment of a patient's clinical conditions. US Federal law restricts this device to sale by or on the order of a physician.
Contact Details:
In USA:
e: info.us@vivosight.com
t: +1 (408) 504 7391
In the UK: Michelson Diagnostics:
e: info@vivosight.com
t: +44 (0)20 8308 1695
Jon Holmes
Michelson Diagnostics
+44 7711 822985
email us here
Port Wine Birthmarks are a new application of VivoSight, but the scanner is already used in the detection of skin caner - watch this video to understand more.
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.