Post-pandemic, life sciences refocuses digital transformation on strategic challenges and the potential of data
Arkivum publishes research report into the latest data management trends affecting clinical trial sponsors and stakeholders
READING, UNITED KINGDOM, August 4, 2022 /EINPresswire.com/ -- Struck by successive waves of the pandemic, the life sciences industry was forced to refocus its digital transformation strategies in order to deliver frontline treatments to patients, as senior executives report. Now, faced with new emerging threats – from supply chain disruption to the global economic slowdown – the focus of digital transformation is on improving productivity, reducing costs and driving scientific innovation across the entire R&D value chain.
Despite these admirable intentions, new research suggests that inadequate data strategies continue to undermine ambitions for digital transformation in life sciences. Missing files, siloed and inaccessible data, and compromises to the integrity of clinical trial records ultimately stand to obstruct endeavours to improve human health.
Arkivum, a world leader in digital archiving and preservation, in collaboration with EMIG (Ethical Medicines Industry Group), has been tracking senior executives’ views on, and adoption of digital transformation and the progress of clinical data strategies for the past three years.
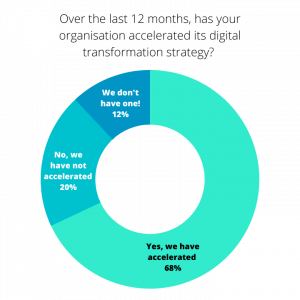
In its 2022 survey, seven in ten (68%) senior executives report that their organisations have accelerated their digital transformation strategies rather than simply persisting with them. Additionally, over half (52%) plan to prioritise improvements in productivity through streamlining processes, whilst 46% will focus on reducing costs.
A further priority for 34% of respondents, with the pharmaceutical sector due to spend over USD 200 billion on research and development (R&D) in the current year, is to improve the management and conduct of one of the most complex, costly and crucial disciplines in life sciences – clinical trials.
Of the 304 senior leaders surveyed, a quarter (24%) are exploring new therapeutics for diseases that are yet to have an effective treatment. 36% say that their organisation is running clinical studies on novel and next-generation treatments such as cell and gene therapies – an increase from 23% in 2021. A growing number of organisations are also conducting trials in repurposing and new indications of existing drugs (14%).
Over the next 12 months, 85% of life sciences organisations say they plan to optimise the conduct of clinical trials, prioritising the adoption of new trial designs and the use of real-world data (RWD), cited by 38%, whilst ensuring compliance with new clinical trial regulations (34%).
As the industry looks to innovate further over the entire R&D cycle – from drug discovery and clinical trials to regulatory reporting – three in four (73%) life sciences organisations say they are likely to invest substantially in technology to facilitate interrogation of clinical data on a large scale. This interrogation will be achieved through the use of advanced technologies and innovations, such as: robotics, artificial intelligence (AI), cognitive automation, real-world evidence (RWE), digital health and telemedicine, and the implementation of archiving/digital preservation technology.
Inadequate data strategies: undermining every aspect of digital transformation strategies in life sciences
86% of all life sciences organisations face challenges when running a clinical trial. These range from issues faced when launching a study (36%) to the realities of stewarding data – integration, management, reporting, and locating missing files (34%).
Half (52%) of life sciences organisations go as far as saying that: “Transferring clinical trial data from the clinical trial software compromises the integrity of the clinical trial data and files, thus causing breaches of regulatory compliance.” This will be a particular cause for concern following the introduction earlier this year of Regulation (EU) No 536/2014, which applies to the conduct of clinical trials.
The majority (84%) of respondents to the Arkivum survey agree that: “Storing and preserving clinical data, records and files enhances the value of data and its potential to drive innovation. A hindrance for many (75%) life sciences organisations, however, is the fact that their data – originating in a diversity of formats from disparate sources and environments – often sits in departmental silos. The cost of storing data (sometimes running to petabytes) in a fit-for-purpose digital archive is perceived as too high. Clinical data and files are therefore housed in inflexible storage solutions that are often difficult to access, and data cannot therefore be ingested and integrated from multiple sources.
Currently, one in four (23%) leave their clinical trial data in their licensed clinical trial software system. These systems are not designed as dedicated solutions for long-term archiving and digital preservation, so this practice could expose organisations to regulatory risk when it comes to data inspections, and to the broader risk of losing access to important and commercially valuable data. Nor is the practice cost-effective.
Chris Sigley, CEO of Arkivum, commented:
“Today, if you ask senior commercial executives in life sciences about their strategies for preserving digital clinical data, three out of four would describe their organisation’s ability to access clinical data, files and records as ‘good’. By contrast, seven in ten specialists in clinical research would describe it as ‘extremely inadequate’ or ‘very inadequate’.
“By no means does digital preservation of clinical data – even at a petabyte scale – have to be prohibitively expensive. It must, however, be done properly. The archiving and preservation of clinical data is not merely an end process. Digital preservation needs to rise up the board agenda and to become ingrained in the psyche of life sciences if the industry is to maximise its capacity to improve human health.”
About the survey
The third annual Arkivum survey: Transforming the Future of Clinical Data, was conducted in April 2022 by Arkivum, in collaboration with the Ethical Medicines Industry Group (EMIG).
The 305 senior representatives surveyed have responsibility for/knowledge of clinical trials and hold senior and director-level positions in the following functions: General and senior management; R&D; Medical Affairs; Quality Assurance; Manufacturing; Regulatory; Clinical; Operations, and Commercial. These senior executives, represent: 171 pharmaceutical and biotechnology companies, 27 health technology companies, 24 healthcare companies, 34 Contract Research Organisations, 34 Contract Manufacturing Organisations, 15 Clinical Research Investigators.
Tom Lynam
Arkivum
+ +44 7990038383
email us here
Visit us on social media:
Twitter
LinkedIn